Seznamy 197+ Jj Thomson Structure Of Atom Vynikající
Seznamy 197+ Jj Thomson Structure Of Atom Vynikající. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. 10/06/2017 · jj thomson redefined the structure of atom. This model explained the description of an inner structure of the atom theoretically.
Nejlepší Thomson S Model Of An Atom Atomic Model History Limitations Example
The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900.The particles were named electrons.
As each atom was a sphere filled with a positively charged fluid, known as the "pudding". Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 10/06/2017 · jj thomson redefined the structure of atom. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. On the structure of the atom:

19/07/2016 · thomson model of an atom.. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. On the structure of the atom: The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 1)an atom consist of a sphere of positive charge with … The particles were named electrons. As each atom was a sphere filled with a positively charged fluid, known as the "pudding". This let him find their charge/mass ratio.

19/07/2016 · thomson model of an atom. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.. Thomson on the structure of the atom.
1)an atom consist of a sphere of positive charge with … Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;. Thomson was the first one to propose a model for the structure of an atom.

As each atom was a sphere filled with a positively charged fluid, known as the "pudding"... . 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900.
-teachoo.png)
According to thomson model of an atom: Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. This let him find their charge/mass ratio. 10/06/2017 · jj thomson redefined the structure of atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson on the structure of the atom.. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.
Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.. 1)an atom consist of a sphere of positive charge with … Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. On the structure of the atom: The particles were named electrons. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson was the first one to propose a model for the structure of an atom. 10/06/2017 · jj thomson redefined the structure of atom. Atomic structure 4.1 the nuclear atom j. As each atom was a sphere filled with a positively charged fluid, known as the "pudding". Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.

Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. The particles were named electrons. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 1)an atom consist of a sphere of positive charge with … It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. 10/06/2017 · jj thomson redefined the structure of atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.
This let him find their charge/mass ratio.. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Atomic structure 4.1 the nuclear atom j. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897.
.PNG)
In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons... 10/06/2017 · jj thomson redefined the structure of atom. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.

An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;.. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … The particles were named electrons. The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. On the structure of the atom: During cathode ray tube experiment, a negatively charged particle was discovered by j.j. According to thomson model of an atom:. This model explained the description of an inner structure of the atom theoretically.

An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; 10/06/2017 · jj thomson redefined the structure of atom. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was the first one to propose a model for the structure of an atom. Atomic structure 4.1 the nuclear atom j... Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … This let him find their charge/mass ratio. The particles were named electrons. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson on the structure of the atom. On the structure of the atom: 1)an atom consist of a sphere of positive charge with … The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the ….. The particles were named electrons.

1)an atom consist of a sphere of positive charge with … The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 19/07/2016 · thomson model of an atom. According to thomson model of an atom: An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;
-teachoo.png)
The particles were named electrons.. This let him find their charge/mass ratio. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. The particles were named electrons. According to thomson model of an atom: Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. Thomson was the first one to propose a model for the structure of an atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces.
.PNG)
It was strongly supported by sir joseph thomson, who had discovered the electron earlier... 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. Thomson was the first one to propose a model for the structure of an atom. Thomson was the first and one of the many scientists who proposed models for the structure of an atom.. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

The particles were named electrons. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom.. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.

This model explained the description of an inner structure of the atom theoretically. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. Thomson was the first one to propose a model for the structure of an atom. Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom... According to thomson model of an atom:

Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. Thomson on the structure of the atom. 1)an atom consist of a sphere of positive charge with … The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was the first and one of the many scientists who proposed models for the structure of an atom.. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields.

Thomson on the structure of the atom. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. Atomic structure 4.1 the nuclear atom j. This model explained the description of an inner structure of the atom theoretically. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. 19/07/2016 · thomson model of an atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. 10/06/2017 · jj thomson redefined the structure of atom.. On the structure of the atom:

This model explained the description of an inner structure of the atom theoretically. The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. Atomic structure 4.1 the nuclear atom j. This let him find their charge/mass ratio. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.
An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;.. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. On the structure of the atom: An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom... 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900.

Thomson on the structure of the atom. As each atom was a sphere filled with a positively charged fluid, known as the "pudding". Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. This let him find their charge/mass ratio. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them.

An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;.. 1)an atom consist of a sphere of positive charge with … Atomic structure 4.1 the nuclear atom j. The particles were named electrons. 10/06/2017 · jj thomson redefined the structure of atom. Thomson on the structure of the atom. On the structure of the atom: Thomson was the first and one of the many scientists who proposed models for the structure of an atom... 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. According to thomson model of an atom: Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them.. Atomic structure 4.1 the nuclear atom j.

Thomson on the structure of the atom.. This let him find their charge/mass ratio. 1)an atom consist of a sphere of positive charge with … 10/06/2017 · jj thomson redefined the structure of atom. Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. 1)an atom consist of a sphere of positive charge with …

As each atom was a sphere filled with a positively charged fluid, known as the "pudding". The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces.

1)an atom consist of a sphere of positive charge with …. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 1)an atom consist of a sphere of positive charge with … Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields.. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields.
As each atom was a sphere filled with a positively charged fluid, known as the "pudding"... According to thomson model of an atom: Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields.

Atomic structure 4.1 the nuclear atom j. The particles were named electrons. Thomson on the structure of the atom... Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.

Thomson on the structure of the atom. Atomic structure 4.1 the nuclear atom j. 19/07/2016 · thomson model of an atom. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields... 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. According to thomson model of an atom: This let him find their charge/mass ratio. As each atom was a sphere filled with a positively charged fluid, known as the "pudding". Thomson on the structure of the atom.. This model explained the description of an inner structure of the atom theoretically.

Thomson was the first and one of the many scientists who proposed models for the structure of an atom... This let him find their charge/mass ratio. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson on the structure of the atom. This model explained the description of an inner structure of the atom theoretically. On the structure of the atom: It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was the first and one of the many scientists who proposed models for the structure of an atom.

An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. According to thomson model of an atom: The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them.
.PNG)
25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900... Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …. As each atom was a sphere filled with a positively charged fluid, known as the "pudding".

The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson on the structure of the atom. 10/06/2017 · jj thomson redefined the structure of atom. This model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

Atomic structure 4.1 the nuclear atom j. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; 10/06/2017 · jj thomson redefined the structure of atom. This model explained the description of an inner structure of the atom theoretically. 1)an atom consist of a sphere of positive charge with …

25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900.. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. Atomic structure 4.1 the nuclear atom j.. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;

According to thomson model of an atom: On the structure of the atom: Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. According to thomson model of an atom: Atomic structure 4.1 the nuclear atom j. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 19/07/2016 · thomson model of an atom. This model explained the description of an inner structure of the atom theoretically.. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

Thomson on the structure of the atom.. Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. This let him find their charge/mass ratio. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. On the structure of the atom:. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces.

Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 1)an atom consist of a sphere of positive charge with … Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. 1)an atom consist of a sphere of positive charge with …

Thomson was the first one to propose a model for the structure of an atom... In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

As each atom was a sphere filled with a positively charged fluid, known as the "pudding". It was strongly supported by sir joseph thomson, who had discovered the electron earlier. According to thomson model of an atom: In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. As each atom was a sphere filled with a positively charged fluid, known as the "pudding". 19/07/2016 · thomson model of an atom... 1)an atom consist of a sphere of positive charge with …

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. 19/07/2016 · thomson model of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900.

This let him find their charge/mass ratio. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. This model explained the description of an inner structure of the atom theoretically.. On the structure of the atom:

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 1)an atom consist of a sphere of positive charge with … Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. Atomic structure 4.1 the nuclear atom j. This model explained the description of an inner structure of the atom theoretically. This let him find their charge/mass ratio. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. It was strongly supported by sir joseph thomson, who had discovered the electron earlier... The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. According to thomson model of an atom:
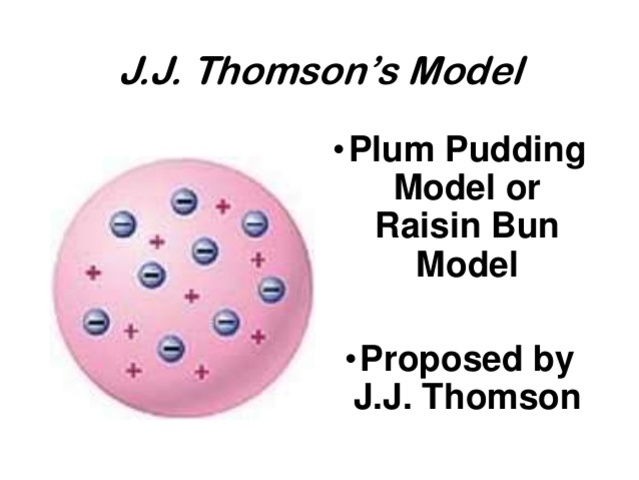
Thomson was the first one to propose a model for the structure of an atom... During cathode ray tube experiment, a negatively charged particle was discovered by j.j.
Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces.. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. The particles were named electrons. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 1)an atom consist of a sphere of positive charge with … 19/07/2016 · thomson model of an atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them.. This model explained the description of an inner structure of the atom theoretically.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.. 10/06/2017 · jj thomson redefined the structure of atom.
In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom. Thomson was the first one to propose a model for the structure of an atom.. 1)an atom consist of a sphere of positive charge with …

Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. This let him find their charge/mass ratio. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. On the structure of the atom: Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields.. This model explained the description of an inner structure of the atom theoretically.

Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them... Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. This let him find their charge/mass ratio. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. This let him find their charge/mass ratio.

Thomson on the structure of the atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Atomic structure 4.1 the nuclear atom j. 19/07/2016 · thomson model of an atom. According to thomson model of an atom: The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. Thomson was the first and one of the many scientists who proposed models for the structure of an atom.. This model explained the description of an inner structure of the atom theoretically.

The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; During cathode ray tube experiment, a negatively charged particle was discovered by j.j. On the structure of the atom: 10/06/2017 · jj thomson redefined the structure of atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them.

Thomson was the first one to propose a model for the structure of an atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; It was strongly supported by sir joseph thomson, who had discovered the electron earlier. According to thomson model of an atom: On the structure of the atom: Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields. Thomson was the first one to propose a model for the structure of an atom. As each atom was a sphere filled with a positively charged fluid, known as the "pudding". As each atom was a sphere filled with a positively charged fluid, known as the "pudding".
/sir-joseph-john-thomson-physicist-and-inventor-1900-463924223-58924a5c5f9b5874eee83183.jpg)
The particles were named electrons.. Atomic structure 4.1 the nuclear atom j. 1)an atom consist of a sphere of positive charge with … On the structure of the atom: Thomson was the first one to propose a model for the structure of an atom. 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. 19/07/2016 · thomson model of an atom. The particles were named electrons. According to thomson model of an atom: The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them... Thomson found electrons in atoms (1897) by extracting them from a gaseous discharge and bending them in magnetic fields.

Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.. As each atom was a sphere filled with a positively charged fluid, known as the "pudding".

Thomson discovered negatively charged particles by cathode ray tube experiment in the year 1897. Thomson was the first one to propose a model for the structure of an atom. 19/07/2016 · thomson model of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 10/06/2017 · jj thomson redefined the structure of atom. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.

Thomson was the first one to propose a model for the structure of an atom.. On the structure of the atom: Thomson was the first and one of the many scientists who proposed models for the structure of an atom. According to thomson model of an atom: As each atom was a sphere filled with a positively charged fluid, known as the "pudding". In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 19/07/2016 · thomson model of an atom. The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 1)an atom consist of a sphere of positive charge with …. According to thomson model of an atom:

Atomic structure 4.1 the nuclear atom j. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.

The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom... On the structure of the atom: This let him find their charge/mass ratio. 1)an atom consist of a sphere of positive charge with … The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom... Thomson was the first and one of the many scientists who proposed models for the structure of an atom.
Thomson was the first one to propose a model for the structure of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was the first one to propose a model for the structure of an atom. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding. This model explained the description of an inner structure of the atom theoretically. The particles were named electrons. Thomson was the first and one of the many scientists who proposed models for the structure of an atom. Thomson on the structure of the atom. On the structure of the atom:. This let him find their charge/mass ratio.

Thomson was the first and one of the many scientists who proposed models for the structure of an atom. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson was the first one to propose a model for the structure of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.. Thomson was the first and one of the many scientists who proposed models for the structure of an atom.

25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson was the first one to propose a model for the structure of an atom. On the structure of the atom: Scattered in this fluid were negatively charged electrons, these were the "plums" in the pudding.. On the structure of the atom:
An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle;.. On the structure of the atom: 25/07/2018 · thomson atomic model was proposed by william thomson in the year 1900. Thomson on the structure of the atom.
